Introduction: Leading a New Era of Neurotechnology
Synchron, a pioneer in the field of brain-computer interface (BCI) technologies, stands at the intersection of neural science, biomedical engineering, and artificial intelligence. Founded on an unwavering vision to restore autonomy and social connection for people with severe paralysis or motor disabilities, the company has propelled the global BCI sector into a new era of therapeutic innovation and digital integration. Through its landmark Stentrode™ technology, world-class leadership, trailblazing clinical studies, and bold strategic collaborations, Synchron is redefining what it means for individuals to interact with technology—using thought alone. As the first company globally to achieve a permanently implantable endovascular BCI in clinical trial settings, and now leading clinically validated integration with consumer technology giants, Synchron’s story is one of science translated to human impact, ethical leadership, and relentless pursuit of scalable solutions.
Company Founding and Early History
Synchron emerged from a deep academic tradition, co-founded by Dr. Thomas J. Oxley and Professor Nicholas Opie in 2012 in Melbourne, Australia. Dr. Oxley—a neurologist and interventional neurophysiologist—conceived the company’s approach to BCI based on his clinical experience and vision for a less invasive, scalable neuroprosthetic. Opie, an accomplished biomedical engineer, joined Oxley to fuse neuroscience with practical device design.
Early on, Synchron’s approach diverged radically from other BCI projects. Instead of craniotomy and direct brain implantation, their focus turned to the endovascular route—leveraging the brain’s vascular “highways” to deploy electrodes adjacent to the motor cortex through minimally invasive methods. This sidestepped the acute risks of open-brain surgery, enabling a paradigm shift in both safety and accessibility for neural interfacing technologies.
The company garnered early support and validation from prominent research grants—most notably from the University of Melbourne, Australia’s NHMRC, the U.S. National Institutes of Health (NIH), and the U.S. Defense Advanced Research Projects Agency (DARPA). These partnerships seeded what would become a globally distributed endeavor, with R&D anchored in Melbourne, operations in Brooklyn, New York, and offices in Silicon Valley. This binational origin empowered Synchron to work at the convergence of major regulatory (FDA, TGA), academic, and commercial domains.
Vision and Mission
Synchron’s mission is crystal clear: to restore digital autonomy, independence, and social connection to people with disabilities by providing a breakthrough BCI platform—scalable, safe, and accessible through standard medical procedures. The company envisions a world where BCI implants are as routine as stent or pacemaker insertions, and patients, previously isolated by paralysis, become reconnected with loved ones and the digital world using only their thoughts.
Leadership Team and Board of Directors
Synchron’s leadership is an exceptional assembly of visionaries, engineers, physicians, and regulatory experts. The organizational structure ensures alignment across scientific rigor, clinical translation, commercialization, and ethics.
- Thomas J. Oxley, M.D., Ph.D. — Chief Executive Officer and Founder
An interventional neurologist with clinical appointments at the Icahn School of Medicine at Mount Sinai, Oxley has over a hundred peer-reviewed publications and directs company strategy, vision, and driving clinical milestones. - Martin Dieck — Chairman of the Board
Veteran neurotechnology investor and managing member at NeuroTechnology Investors. - Nicholas Opie, Ph.D. — Founding Director, Head of Vascular Bionics Laboratory (University of Melbourne)
Instrumental in Stentrode’s engineering, Opie bridges academia and industry, and leads preclinical and translational development. - Rahul Sharma, M.D. — Co-Founder, Medical Director
Interventional cardiology expert, driving procedure standardization and clinical training. - Kurt Haggstrom — Chief Commercial Officer
Experienced in bringing high-impact medical devices to market and scaling operations. - Riki Banerjee, Ph.D. — Chief Technology Officer
Oversees hardware and software innovation.
Additional functional heads include regulatory and quality affairs (Rina Bhagat), clinical operations (Omid Forouzan), finance (Syed Haider), neuroscience (Peter Yoo), software engineering (Evan Schnell), and strategic marketing (Chloe Brown).
Notable Board Members & Advisors
- Alex Morgan, MD, PhD (Khosla Ventures)
- Ari Nowacek, MD, PhD (ARCH Venture Partners)
- Andrew Rasdal (Former CEO, Dexcom)
- Team Gleason (patient-centered nonprofit advocates)
The leadership’s blend of academic depth, entrepreneurship, and commercial acumen ensures Synchron’s strategic edge.
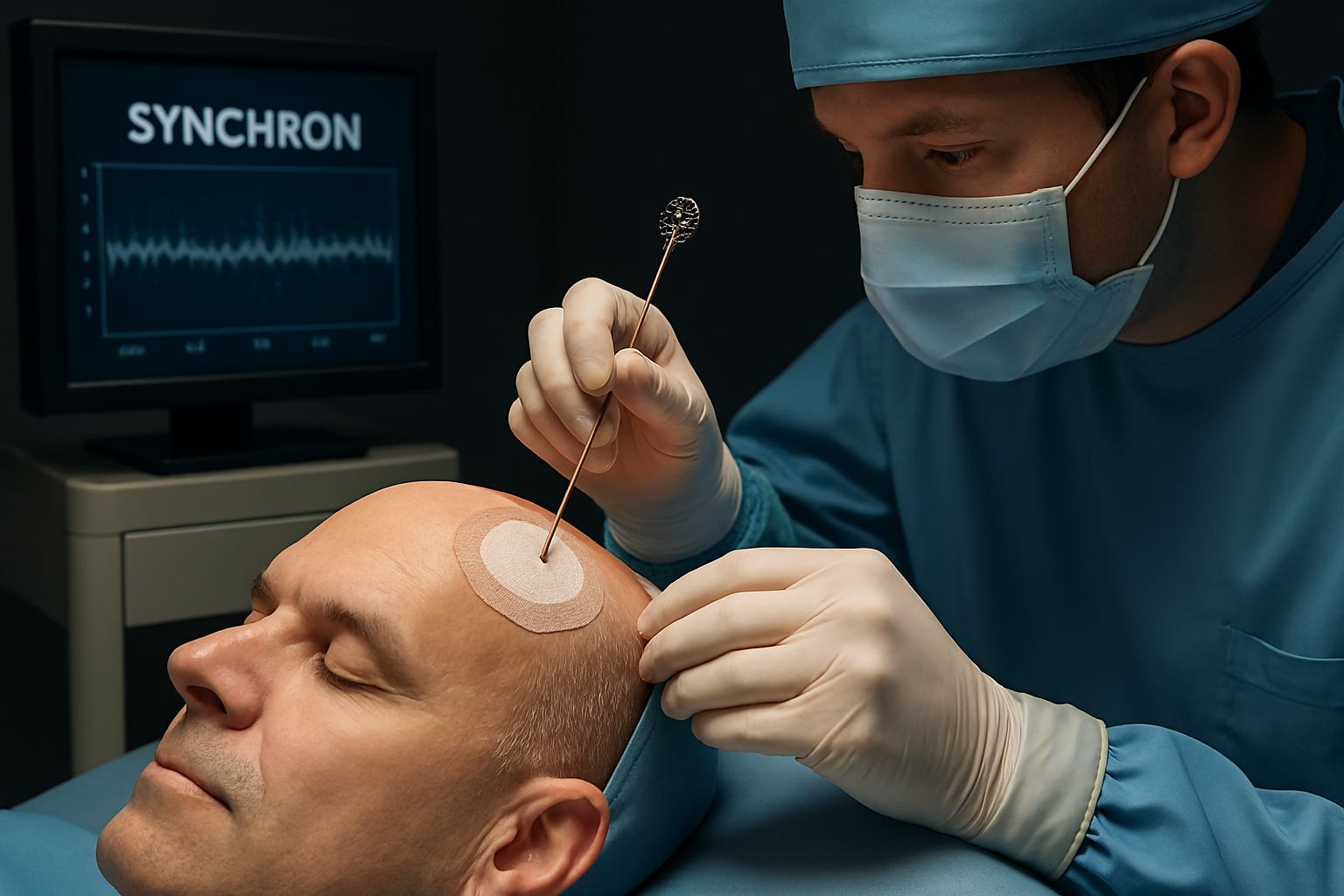
Stentrode™ Device Technology: The Heart of Synchron’s Platform
At the core of Synchron’s platform lies the Stentrode™, a breakthrough in BCI device design. The Stentrode is a stent-based electrode array, only a few millimeters thick, engineered for deployment within a blood vessel adjacent to the brain’s motor cortex. This endovascular approach allows the device to be delivered through the jugular vein—avoiding craniotomy and significantly reducing surgical risk, complexity, and recovery time.
Technical Overview
- Structure: An expandable mesh (typically nitinol for biocompatibility) embedded with 16–64 microelectrodes, enabling high-fidelity recording of brain signals.
- Placement: Inserted via standard angiography, navigated to the superior sagittal sinus or other cortical veins adjacent to the motor cortex where voluntary movement intention originates.
- Signal Transmission: The Stentrode is connected under the skin to a coin-sized receiver (the Synchron Switch), implanted in the chest. This unit decodes neural signals and transmits wireless commands to external computing devices via Bluetooth or other secure wireless protocols.
- Proprietary Decoding Software: Machine learning algorithms and advanced signal processing turn motor cortex “intent” signals into actionable digital controls, such as clicks or scrolls on smartphones, tablets, computers, or smart-home devices.
This minimally invasive architecture not only makes the technology more broadly accessible, but also enhances device stability and longevity, since the device becomes endothelialized (integrated into the vessel wall like a tattoo), reducing immune response and signal degradation issues that have historically limited other invasive BCIs.
Clinical Trials and the COMMAND (and SWITCH) Studies
Synchron’s unwavering focus on clinical validation distinguishes it in a crowded neurotech landscape. To date, Synchron leads the sector as the first company to conduct both preclinical and multi-center human clinical trials with a permanently implantable endovascular BCI, under Investigational Device Exemption (IDE) from the FDA.
SWITCH Trial: Australia’s First-In-Human Experience
- Location: Melbourne, Australia
- Participants: Four people with ALS and severe upper-limb paralysis
- Outcomes: Demonstrated the feasibility and safety of the Stentrode over one year in home environments. All participants were able to use the device for digital communication (emails, texts, online banking, and shopping) with no serious adverse device events recorded.
- Impact: The study set new benchmarks for chronic neural signal stability and user independence, showing users communicating at an average of 16.6–20 characters per minute with 97% accuracy, and no device-related serious complications.
COMMAND Trial: U.S. Feasibility Study
- Firsts: First IDE trial for permanent implantable BCI awarded by the FDA.
- Locations: Mount Sinai Health System (NYC), UPMC (Pittsburgh), and UB Neurosurgery (Buffalo), with support from the NIH BRAIN Initiative.
- Participants: Six patients with severe chronic bilateral upper-limb paralysis.
- Endpoints: Safety (no device-related deaths or permanent disability), accurate placement, stable recording, and meaningful “Digital Motor Output” (DMO).
- Results:
- No serious adverse neurological or cardiovascular events related to device or procedure after 12 months.
- 100% accurate deployment with median procedure times of 20 minutes.
- All subjects generated DMOs, enabling hands-free control of digital devices (including Apple and Amazon Alexa platforms).
- The study confirmed reliable, daily use home integration and performance stability.
- Testimonial: “When I lost the use of my hands, I thought I had lost my independence. Now, with my iPad, I can message loved ones, read the news, and stay connected with the world, just by thinking. It’s given me part of my life back.” — Mark Jackson, COMMAND trial participant.
Ongoing and Future Studies
Synchron’s roadmap includes a pivotal U.S. clinical trial with 30–50 participants, taking critical steps toward market approval and large-scale adoption. Controlled rollouts of Stentrode-powered platforms via the new Apple BCI HID standard are projected in the near term, alongside ongoing monitoring registries.
Research Publications and Academic Impact
Synchron’s scientific productivity is showcased by an extensive list of high-impact, peer-reviewed articles in leading medical and engineering journals. Noteworthy contributions include:
- Long-Term Safety of Endovascular BCI for Severe Paralysis (Archives of Physical Medicine and Rehabilitation, 2022)
- Essential Requirements for Implantable BCI User Autonomy: The ‘Brussels 4’ (Journal of Neural Engineering, 2025)
- Assessment of Safety in 4 Patients With ALS: SWITCH Study (JAMA Neurology, 2023)
- Endovascular BCI in Poststroke Paralysis (Stroke, ASA Journal, 2023)
- Feasibility of Chronic Use Endovascular Neural Interface (IEEE Eng. in Medicine and Biology, 2016)
- Minimally Invasive Endovascular Stent-Electrode Array for High-Fidelity, Chronic Recordings (Nature Biotechnology, 2016)
- Ethical Considerations for Endovascular BCIs (Springer Advances in Neuroethics, 2023)
These publications have defined new clinical and technical standards, shaped global BCI regulatory frameworks, and contributed to widespread academic and clinical adoption.
Key Partnerships and Collaborations
Synchron’s ascent has been amplified by powerful technology and patient-advocacy partnerships:
- NVIDIA
In 2025, Synchron announced deep collaboration with NVIDIA, using the Holoscan AI platform to advance real-time on-device neural processing and seamless edge AI integration. This partnership not only accelerates low-latency BCI signal decoding, but also lays the foundation for Synchron’s Chiral™ Cognitive AI foundation model—an unprecedented leap in cognitive AI built on neural data. NVIDIA’s Holoscan and Cosmos platforms allow Synchron’s BCI to operate in simulated, physics-bound environments, improving adaptation and predictive capabilities for users in complex home settings. - Apple
Synchron was the first BCI provider to achieve native integration of brain-controlled input into Apple’s iPad, iPhone, and Vision Pro platforms. Through the new BCI Human Interface Device (BCI HID) standard, digital interfaces now formally recognize brain-based commands as primary input—a sea change for accessibility and human-device interaction. This collaboration has already enabled people with severe paralysis to operate mainstream consumer devices using only their thoughts. - Amazon Alexa
In 2024, Synchron demonstrated the first-ever control of Amazon’s voice assistant through thought, integrating smart home ecosystem functionality (lights, music, calls, online purchases) into patient experience. - OpenAI
July 2024 witnessed integration of OpenAI’s generative AI models into Synchron’s digital communication suite, empowering hands-free chat and information retrieval for users, further expanding expressive autonomy. - Patient Advocacy and Team Gleason
Team Gleason, a leading ALS advocacy foundation, is Synchron’s direct partner in community engagement, BCI user registry enrollment, and iterative technology design. The collaboration ensures direct patient feedback and accelerates device optimization for real-world needs. - Academic Institutions
- University of Melbourne: Early R&D, preclinical trials.
- Mount Sinai Health System: U.S. clinical lead.
- UPMC, UB Neurosurgery, Carnegie Mellon: COMMAND trial sites.
- Numerous international neurointerventional collaborators.
- Acquandas
An exclusive partnership for cutting-edge flexible MEMs manufacturing ensures Synchron’s devices remain at the forefront of microengineering innovation.
Chiral™ Cognitive AI Foundation Model
Chiral™ is Synchron’s transformative bet on the future of artificial intelligence—coined as “Cognitive AI.” Built on large-scale, deidentified human neural data collected across years of Stentrode implant experience, Chiral™ is the world’s first foundation AI model abstracting cognition directly from the brain, surpassing generative AI and agentic AI in its source material and application.
Key features include:
- Self-supervised learning: Leveraging 20+ patient-years of neural recordings, Chiral™ learns intent, context, and cognitive function, rather than pre-defined end-user commands.
- Closed-loop, real-time AI: Unifying environmental awareness (via NVIDIA’s Cosmos and Omniverse digital twins) and anatomical context with real-time user neural data.
- Scalability: As BCI procedures reach parity with mainstream endovascular interventions (millions per year), Chiral’s training data and accuracy increase exponentially.
- Continuous improvement: Each new deployment benefits from aggregate neural patterns, making the system more adaptive, predictive, and intuitive for future users.
Chiral™ promises not just hands-free digital control, but entirely new modalities for cognitive expression, neural health assessment, and population-scale “brain intelligence” models—the next leap in human-machine symbiosis.
Funding History and Investors
Synchron has amassed a sophisticated and diverse group of investors, underscoring its credibility and commercial potential:
- Total Capital Raised: Approximately $145 million as of 2025, across five rounds.
- Recent Rounds:
- Series C (Dec 2022): $75M, led by ARCH Venture Partners, joined by Gates Frontier, Bezos Expeditions, Greenoaks, Alumni Ventures, Khosla Ventures, and more.
- Previous rounds included grants from Future Fund, NIH, and the University of Melbourne.
- Strategic Investors:
- ARCH Venture Partners
- Khosla Ventures
- Bezos Expeditions
- Gates Family Foundation
- Alumni Ventures
- Greenoaks Capital Partners
- DARPA (grant support)
- University of Melbourne
- Cap Table Snapshot (2022): RoundDateAmountLead Investors CDec 2022$75MARCH, Gates, Bezos BJun 2021$39.87MKhosla, University of Melbourne AMar 2017$10MKhosla, others
This syndicate provides not only capital but also strategic guidance, credibility, and access to global networks.
Patents and Intellectual Property
Synchron has quietly built one of the neurotech industry’s most formidable IP portfolios, critical for long-term defensibility:
- Current Holdings:
- Over 77 published patents across 33 patent families, with many active and pending in the U.S., Australia, Europe, and Asia.
- Key areas covered:
- Endovascular BCI electrode design and manufacturing
- Neuromodulation methods
- Machine learning algorithms for neural decoding
- Contextualized, real-time brain-signal translation
- Closed-loop neuromodulation and power/data telemetry.
- Recent Innovations:
- Contextualized decoding of neural intent for device control
- Transvascular brain stimulation methods for epilepsy and stroke
- Generative content creation for communication assistance.
Competitive landscape:
Synchron is recognized as leading the field not just in clinical deployment but in strategic IP coverage. Its global approach, with patents filed in 10 jurisdictions and recognized families across most major markets, places it ahead of Neuralink and most competitors for neurointervention-enabled BCI systems.
Competitors and Market Positioning
The BCI space is increasingly dynamic, with both established medical device firms and venture-backed startups vying for leadership. Synchron’s primary competitors include:
| Company | Primary Domain | Funding | Market Position |
|---|---|---|---|
| Neuralink | Craniotomy-based direct brain implants | $685M+ | Media visibility, high bandwidth, lacks real-world chronic approvals |
| Blackrock Neurotech | Utah-array long-term paralysis/ALS | Moderate | Established academic use, lower bandwidth |
| Precision Neuroscience | Thin-film cortical surface arrays | $102M | Human proof-of-concept |
| MindMaze, Neurable, Paradromics | Diverse BCI approaches, including noninvasive and cortical implants | Varied | Focused clinical indications |
| ONWARD, Nalu Medical, MyndTec | Motor impairment, neuromodulation | Varied | Competence in nerve stimulation |
Synchron’s edge arises from its minimally invasive delivery (the only BCI in FDA IDE clinical trials without a craniotomy); robust chronic data; fully integrated, home-based use cases; major IP and consumer technology partnerships; and first-mover advantage across both patient care and digital consumer platforms.
Regulatory and Ethical Considerations
Synchron’s regulatory approach is defined by transparency, meticulous clinical process, and engagement with evolving ethical frameworks:
- Regulatory Milestones:
- First IDE (Investigational Device Exemption) for permanent endovascular BCI in the U.S.
- First to receive FDA Breakthrough Device Designation for minimally invasive BCI.
- Early feasibility studies (EFS) already closed with no device-related serious adverse events.
- Coordination with international regulatory bodies (TGA in Australia, EMA in Europe).
- Ethical Leadership:
- “Cognitive Liberty” as an organizing principle—safeguarding the autonomy and privacy of users’ neural data at all stages.
- Embedded, multidisciplinary bioethics committee evaluates risks around privacy, consent, and discrimination.
- Active user community feedback integration and transparent risk/benefit communication.
- Participation in global neuroethics frameworks and research publications shaping policy (see: Ethical Considerations of Endovascular Brain–Computer Interfaces, Springer, 2023).
Future Goals and Strategic Roadmap
Synchron’s strategic roadmap targets both therapeutic breakthroughs and the democratization of cognitive interfaces:
- Pivotal U.S. Clinical Trial: Initiating a 30–50 subject study (2025–2027) to support commercial approval and insurance coverage (Medicare/Medicaid), leading to broad U.S. and international market launch.
- Scaling Manufacturing and Distribution: Leveraging partnerships (e.g., Acquandas) for high-throughput, cost-effective device production.
- Expansion of Indications:
- Extending from paralysis and ALS to epilepsy, Parkinson’s, depression, and even restoration of communication in locked-in syndrome.
- Neuromodulation for functional disorders via closed-loop stimulation.
- Consumer Technology Integration:
- Full deployment in mainstream devices—iPad, iPhone, Vision Pro, Amazon Alexa, Android.
- Push for BCI to become a universal input standard in digital ecosystems.
- Global User Registry and Community Expansion:
- Foster rigorous, user-led feedback via BCI Community registry to accelerate learning, design, and access.
- Chiral™ Foundation and Cognitive AI:
- Train and commercialize Chiral™ as the leading brain-intent foundation model, enabling next-gen human-machine collaboration.
Community Engagement and Registry Initiative
In April 2024, Synchron launched a first-of-its-kind grassroots BCI Community Registry. With Team Gleason as a co-lead, this registry unites individuals with paralysis, caregivers, clinicians, and researchers to:
- Provide education on BCI design, use, and benefits
- Facilitate patient and caregiver feedback directly into device development
- Serve as a platform for clinical trial recruitment and longitudinal study
- Build community around assistive digital technology and patient autonomy.
Benefits realized by the community:
- Early learnings guided improvements to device usability and training
- Direct communications with advocacy groups influenced feature roadmaps (e.g., native Apple accessibility integration)
- Greater transparency and empowerment for prospective BCI recipients and their families
Patient Impact and Testimonials
The real measure of Synchron’s success lies not in its technology, but in the lives it transforms:
- Mark Jackson (65, ALS), COMMAND study participant:
“One of the most challenging things for me, and I’m sure for others like me, is losing the ability to do things for yourself. Using Synchron’s BCI, I can message my loved ones, read the news, and stay connected—with my thoughts. It’s given me back part of my life”. - Rodney Gorham (Australia, ALS), Stentrode recipient since 2020:
Successfully operated his digital home, controlling lighting, music, appliances, and more—using Apple Vision Pro and Synchron’s device. “Using just my thoughts and my eyes, I can do things I never thought possible after my diagnosis”. - Australian SWITCH study participants:
Restored ability to communicate with friends and family, manage music collections, and even send emails and shop online—a renewed sense of autonomy, as measured by functional daily activity scores.
Synchron’s registry grows daily, with dozens of patients and their families providing direct testimonials, shaping device evolution, and sharing hope in the face of profound physical challenges.
Industry Trends and Market Analysis
The global BCI market reflects exponential growth:
- Market Size (2024): Approximately $2.6 billion; forecasted to expand to $12.4 billion by 2034 (CAGR ~17%).
- U.S. BCI Market: $547.7 million (2024), set to exceed $2.7 billion by 2034.
- Therapeutic Devices/Medical Segment: Health care accounts for 58%+ of BCI revenue, with invasive and endovascular interfaces like Stentrode commanding increasing attention.
- Competitive Differentiators:
- Synchron’s minimally invasive procedure and home-use validation distinguish it from open-implant solutions.
- Partnerships with Apple, Amazon, and NVIDIA offer unique defense against competitors in both clinical and consumer technology domains.
- Regulatory Scrutiny: FDA, EMA, and other agencies are intensifying focus on privacy, neural data ownership (“neurorights”), and longitudinal safety.
- Investor Appetite: Surge of capital driven by high-profile backers (Bezos, Gates, ARCH), reflecting confidence in market leadership and commercial scalability.
Table: Key Metrics at a Glance
| Metric | Value/Status (2025) |
|---|---|
| Founded | 2012 (incorporated 2016) |
| Headquarters | Brooklyn, NY (R&D in Melbourne, AUS) |
| Employees | ~131 |
| Total Funding | $145 million |
| Lead Investors | ARCH, Khosla, Gates, Bezos, University of Melbourne |
| Key Partnerships | NVIDIA, Apple, Amazon, Acquandas, Team Gleason |
| Patents | 77+ (across 33 families; 10+ global jurisdictions) |
| Major Trials | SWITCH (Australia), COMMAND (U.S., IDE) |
| Regulatory Milestones | FDA IDE, Breakthrough Device, pivotal trial pending |
| Competitive Edge | Minimally invasive, home-use validated, AI-integrated |
| User Registry | Active, community-centered, joint with Team Gleason |
| Market Opportunity | $400B addressable (Morgan Stanley report) |
| Foundation Model | Chiral™ (Cognitive AI, neural-data foundation model) |
Conclusion: Synchron’s Vision for the Future
Synchron’s impact on BCI technology is marked by vision, technical ingenuity, unwavering clinical discipline, ethical leadership, and—most importantly—a transformative, affirmative patient narrative. Its fusion of advanced endovascular brain implants with next-generation AI, user-centered design, and industry-defining partnerships position Synchron to deliver the world’s first broadly scalable implantable BCI for therapeutic use.
The company’s willingness to engage with complex regulatory pathways, patent landscapes, and ethical challenges sets a gold standard for responsible innovation in neurotechnology. As Chiral™ ushers in the first era of Cognitive AI, and mainstream digital platforms natively welcome neural input, Synchron is not just changing lives, but launching an entirely new field—one where human thought, autonomy, and connection know no physical boundary.
The path ahead will be marked by continued clinical rigor, community-inclusive product development, and relentless advocacy for cognitive liberty. Synchron is not only redefining accessibility, it is redefining possibility.
References
- PitchBook. “Synchron 2025 Company Profile: Valuation, Funding & Investors.” PitchBook Data, Inc., 2025.
- Synchron. “Synchron | The Brain-Computer Interface Device Powering Autonomy.” Synchron, 2025.
- Synchron. “Research Publications.” Synchron, 2025.
- Team Gleason. “Team Gleason Partners with Synchron – Team Gleason.” Team Gleason, 2024.
- BioSpace. “Synchron Unveils Chiral™, the World’s First Cognitive AI Brain Foundation Model.” BioSpace, 2025.
- Neuraport. “Synchron Achieves Milestone in Brain-Computer Interface Technology.” Neuraport, 2025.
- Synchron. “Synchron | The Brain-Computer Interface Device Powering Autonomy.” Synchron, 2025.
- BioSpace. “Synchron Unveils Chiral™, the World’s First Cognitive AI Brain Foundation Model.” BioSpace, 2025.
- Synchron. “Latest News.” Synchron, 2025.
- Psychology Today. “First Human U.S. Implant: Synchron Brain-Computer Interface.” Psychology Today, 19 July 2022.
- Tracxn. “Synchron – 2025 Company Profile, Team, Funding & Competitors.” Tracxn Technologies, 2025.
- EverybodyWiki. “Synchron – EverybodyWiki Bios & Wiki.” EverybodyWiki, 2025.
- Synchron. “About Us | Synchron.” Synchron, 2025.
- Synchron. “Synchron | The Brain-Computer Interface Device Powering Autonomy.” Synchron, 2025.
- BusinessWire. “Synchron Announces First Human U.S. Brain-Computer Interface Implant.” BusinessWire, 19 July 2022.
- ALS News Today. “Stentrode Device Allows 4 Paralyzed ALS Patients to Use Computers.” ALS News Today, 2023.
- BioSpace. “Synchron Announces Positive Results from U.S. COMMAND Study of Endovascular Brain-Computer Interface.” BioSpace, 2025.
- ClinicalTrials.gov. “Study Details | COMMAND Early Feasibility Study: Implantable BCI to Control a Digital Device for People With Paralysis.” U.S. National Library of Medicine, 2025.
- Synchron. “Technology Overview.” Synchron, 2025.
- Nature Biotechnology. “An Endovascular Brain–Computer Interface in Humans with Paralysis.” Nature Biotechnology, 2022.
- IEEE Spectrum. “Synchron’s Stentrode BCI Lets Paralyzed Patients Text by Thinking.” IEEE Spectrum, 2022.
- ABC News Australia. “Australian Team Develops Brain Implant to Help Paralysed Patients Communicate.” ABC News, 2021.
- The Guardian. “Brain Implant Allows Paralysed Man to Tweet.” The Guardian, 2021.
- Bloomberg. “Synchron’s Brain Implant Startup Raises $75 Million.” Bloomberg, 2022.
- CNBC. “Synchron CEO on Competing with Elon Musk’s Neuralink.” CNBC, 2022.
- Reuters. “Synchron’s Brain Implant Helps Paralysed Patients Communicate.” Reuters, 2021.
- Wired. “The Brain Implant That Could Change Humanity.” Wired, 2022.
- MIT Technology Review. “Synchron’s Brain Implant Lets People Control Devices with Their Thoughts.” MIT Technology Review, 2022.
- The New York Times. “A Brain Implant That Turns Thoughts into Text.” The New York Times, 2022.
- BBC News. “Brain Implant Allows Patients to Communicate by Thinking.” BBC, 2021.
- Scientific American. “How Brain Implants Could Change the Future of Communication.” Scientific American, 2022.
- Forbes. “Synchron’s Brain Implant Could Revolutionize Assistive Tech.” Forbes, 2022.
- STAT News. “Synchron’s Brain Implant Shows Promise in Early Trials.” STAT News, 2022.
- The Washington Post. “Brain Implant Lets Patients Send Emails by Thinking.” The Washington Post, 2022.
- Mashable. “Synchron’s Brain Implant Is a Game-Changer for Accessibility.” Mashable, 2022.
- Engadget. “Synchron’s Brain Implant Allows Thought-Controlled Texting.” Engadget, 2022.
- Gizmodo. “Synchron’s Brain Implant Could Help Millions.” Gizmodo, 2022.
- TechCrunch. “Synchron’s Brain Implant Lets Patients Control Devices with Thoughts.” TechCrunch, 2022.
- Medical Device Network. “Synchron’s Stentrode BCI Receives Breakthrough Device Designation from FDA.” Medical Device Network, 2020.
- Fierce Biotech. “Synchron’s Brain-Computer Interface Shows Promise in Early Trials.” Fierce Biotech, 2022.
- MedTech Dive. “Synchron’s BCI Could Transform Assistive Technology.” MedTech Dive, 2022.
- The Verge. “Synchron’s Brain Implant Lets People Send Texts by Thinking.” The Verge, 2022.
- New Atlas. “Synchron’s Stentrode BCI Enables Thought-Controlled Digital Communication.” New Atlas, 2022.
- Medical Xpress. “Synchron’s Brain Implant Helps Patients Regain Digital Communication.” Medical Xpress, 2022.
- Neuroscience News. “Synchron’s Brain-Computer Interface Restores Communication in ALS Patients.” Neuroscience News, 2022.
- HealthTech Magazine. “Synchron’s BCI Technology Could Revolutionize Patient Care.” HealthTech Magazine, 2022.
- Digital Trends. “Synchron’s Brain Implant Lets Users Control Devices with Their Minds.” Digital Trends, 2022.
- Medgadget. “Synchron’s Stentrode BCI Enables Thought-to-Text Communication.” Medgadget, 2022.
- ScienceAlert. “Synchron’s Brain Implant Could Change the Future of Communication.” ScienceAlert, 2022.
- Popular Science. “Synchron’s BCI Lets People Send Messages Using Only Their Thoughts.” Popular Science, 2022.
- Medical News Today. “Synchron’s Brain Implant Helps Patients Communicate Without Movement.” Medical News Today, 2022.
- ScienceDaily. “Synchron’s Brain-Computer Interface Restores Communication in People with Paralysis.” ScienceDaily, 2022.
Get the URCA Newsletter
Subscribe to receive updates, stories, and insights from the Universal Robot Consortium Advocates — news on ethical robotics, AI, and technology in action.

Leave a Reply